Label Locations
CS 9600 Labels
The following figures are used only to illustrate label locations, the contents of the labels may be different.
Figure 1 - CS 9600 Label Locations
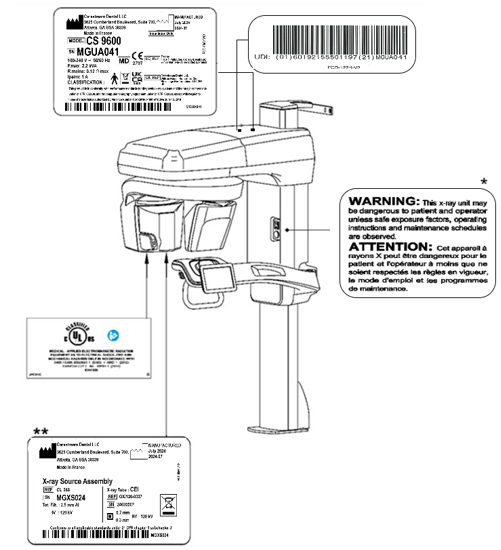
 |
IMPORTANT: * Only for USA this warning appears in the Parameter pane of the Acquisition interface. |
Figure 2—CS 9600 Label Locations (with Scan Ceph Configuration)
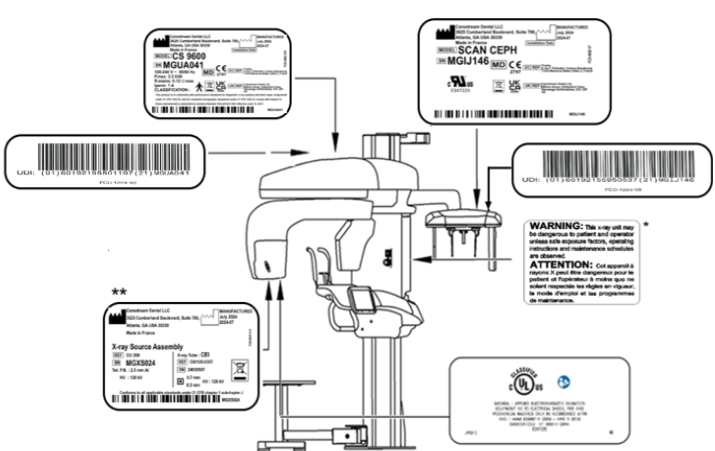
 |
IMPORTANT: * Only for USA this warning appears in the Parameter pane of the Acquisition interface. |
Table 1—Label Definitions
|
Label |
Definition |
|
|
Defines the unit's model |
|
|
Defines the date that the unit was installed |
|
This product is in conformity with performance standards for diagnostic x-ray systems and their major components under 21 CFR 1020.30, and for computed tomography equipment under 21 CFR 1020.33, except with respect to those characteristics authorized by Variance Number FDA-2018-V-1901 effective June 15, 2018 |
Defines the unit's compliance with the US FDA radiation standards |

